Introduction
Rapid industrial development has led to the release of wastewater containing toxic heavy metals, which can accumulate in the human body even at trace levels and cause severe health problems [1–3]. For example, lead (Pb) can interfere with bone biosynthesis, hinder erythrocyte formation by binding to enzymes in bloodstream, and damage multiple organs [4,5]. Cadmium (Cd), known for causing Itai-Itai disease, can induce kidney, nervous system, and skeletal disorders [6,7].
To remove such heavy metals from water, porous solid-state adsorbents such as zeolite, carbon materials, and functional polymers have been widely studied [8–12]. These materials often incorporate soft-base functional groups (e.g., –SH) that can strongly interact with soft-acid metal ions (i.e., heavy metal ions such as Pb2+ and Cd2+) based on the hard-soft acid-base (HSAB) theory [11–13]. However, these traditional adsorbents suffer from several limitations, including poorly controlled pore sizes, challenges in including target functional groups, low adsorption capacities, weak binding affinities, and limited selectivity [14–16].
To overcome these challenges, metal-organic frameworks (MOFs) have emerged as highly tunable porous materials suitable for a wide range of applications, including heterogeneous catalysis, ionic conduction, sensors, gas storage, separation, and adsorption [17–26]. Their designable structures allow for precise control of pore size, surface area, and functionality, as well as high thermal and chemical stability [27–30]. Among them, Zr-based MOFs (e.g., UiO-66, UiO-67, MOF-808, NU-1000) have attracted particular interest because of their exceptional stability and their ability to introduce additional functional groups via Zr6 metal nodes without compromising structural integrity [31–34]. For instance, sulfamic acid has been grafted onto MOF-808 (which has pore sizes of 11.8 Å and 18.4 Å) through the six unsaturated coordination sites of the Zr6 nodes, enhancing acidity and improving proton conductivity under humidity conditions [35].
In this work, we first grafted N-acetyl-L-cysteine (AcC) onto MOF-808 (Zr6O4(OH)4(BTC)2X6, where H3BTC=1,3,5-bezenetricarboxylic acid and X− represents formate or OH−) through ligand exchange at the X− sites. AcC contains thiol (–SH) groups that serve as active sites for capturing soft-acid metal ions (Pb2+ and Cd2+), derived from the L-cysteine (Cys) moiety, which is widely used in medicines and dietary supplements (Fig. 1). Its aliphatic fragment improves solubility in methanol, facilitating incorporation into the Zr6 nodes. This resulting AcC@MOF-808 contained five AcC ligands per Zr6 cluster (corresponding to 83% of the available sites) and retained free –SH groups after modification.
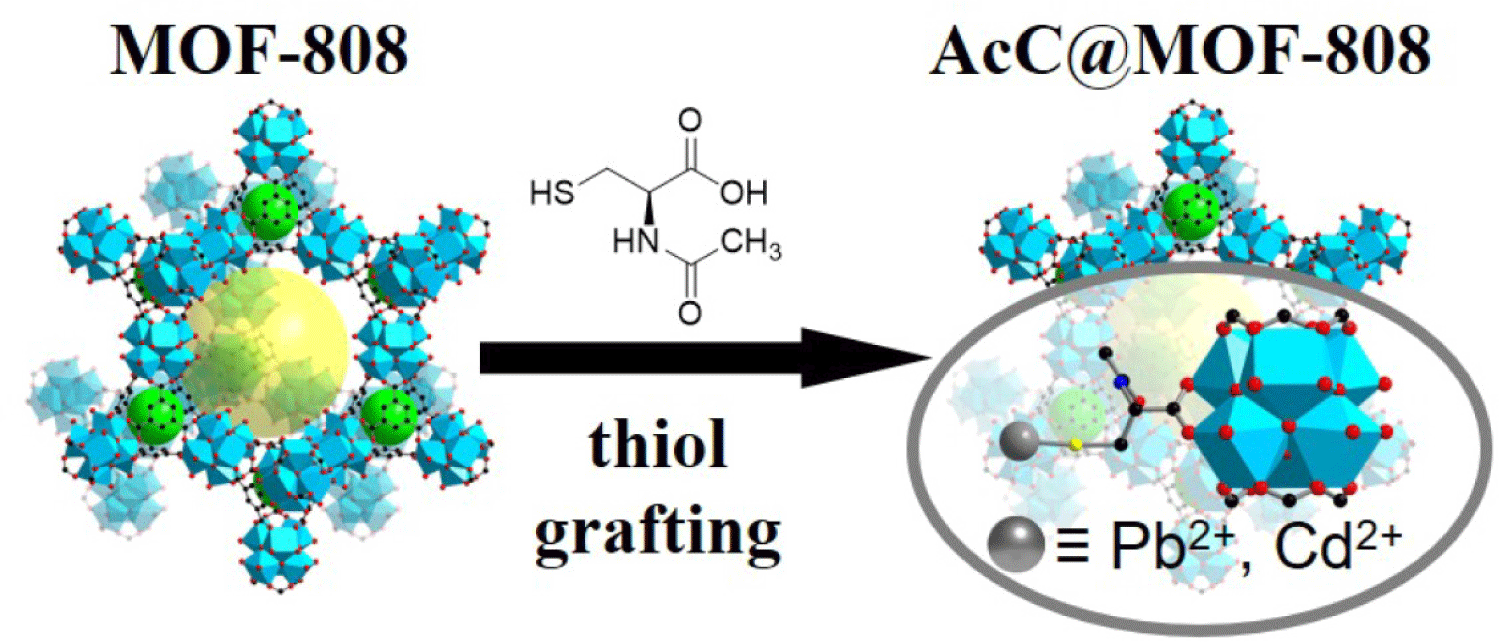
Through these accessible thiol groups, together with the aliphatic and acetyl functionalities introduced within the MOF pores, AcC@MOF-808 effectively adsorbed Pb2+ and Cd2+ while maintaining its crystallinity and morphology, confirming its stability as a heterogeneous solid-state adsorbent. The removal efficiencies for Pb2+ and Cd2+ were both 99%, significantly higher than those for other divalent alkali and transition metal ions (Mg2+, Ca2+, and Zn2+). Notably, AcC@MOF-808 removed 99% for Pb2+ within seven minutes. Owing to this rapid adsorption behavior, kinetic studies revealed that the process followed a pseudo-second order model, with rate constant (k) values of 6.6×103 g mg−1 min−1 and 1.1×103 g mg−1 min−1 for Pb2+ and Cd2+, respectively, which are competitive with those of other thiol-based adsorbents. The maximum adsorption capacities were 231 mg g-1 for Pb2+ and 109 mg g-1 for Cd2+.
This work demonstrates the successful incorporation of an eco-friendly amino acid derivative (AcC) into MOF-808, resulting in a stable, functionalized framework capable of selectively and efficiently removing heavy metals from aqueous systems. Our results highlight the potential of amino acid-modified MOFs as robust and sustainable adsorbents for real-world water purification applications.
Methods
Commercially available reagents were purchased in high purity and used without further purification. ZrOCl2·8H2O (99.5%), Pb(NO3)2 (99.999%), Cd(NO3)2·4H2O (99.997%), 1,3,5-benezene tricarboxylic acid (95%), and AcC (99%) were purchased from Sigma-Aldrich. Formic acid (96%) was purchased from Daejung Chemicals & Metals. N,N-dimethylformamide (DMF; 99.5%) and methanol (99.8%) were purchased from Junsei Chemical (Tokyo, Japan).
Powder X-ray diffraction (PXRD) patterns were recorded on a Rigaku D/max-2400 X-ray powder diffractometer (Tokyo, Japan) using Cu-Kα (λ=1.5405 Å) radiation. Fourier transform infrared (FTIR) spectroscopy was recorded as Attenuated Total Reflectance method using a NICOLET iS 10 FT-IR spectrophotometer. 1H nuclear magnetic resonance (NMR) spectra were measured on Bruker model AVANCE III HD, 400 MHz NMR spectrometer. Scanning electron microscopy (SEM) was performed using a FEI model Nova Nano230. High-resolution transmission electron microscopy (HR-TEM) and energy dispersive spectroscopy (EDS) elemental mapping were performed using a JEOL model JEM-2100F (Cs corrector). Inductively coupled plasma mass spectroscopy (ICP-MS) and inductively coupled plasma optical emission spectroscopy (ICP-OES) data was gathered from Perkin Elmer model ELAN DRC-II and Varian model 700-ES. X-ray photoelectron spectroscopy (XPS) was performed using a Thermo Scientific instrument and K-alpha surface analysis. N2 sorption isotherms were measured using a BELSORP-max (BEL Japan, Tokyo, Japan) adsorption system, employing a standard volumetric technique up to saturation pressure N2 sorption isotherms were monitored at 77 K.
MOF-808 was synthesized following a slightly modified literature procedure [35]. In a 100-mL vial, ZrOCl2·8H2O (483 mg, 1.50 mmol) and 1,3,5-benezene tricarboxylic acid (H3BTC; 315 mg, 1.50 mmol) were dissolved in 15.0 mL of DMF along with 15.0 mL of formic acid. The resulting solution was heated in an oven at 100°C for 18 hours. After cooling to room temperature, the white precipitate was collected and washed three times with fresh DMF and four times with methanol (MeOH) by centrifugation. The product was then dried under vacuum at 60°C overnight. The PXRD pattern matched the reported simulated pattern (Fig. 2).
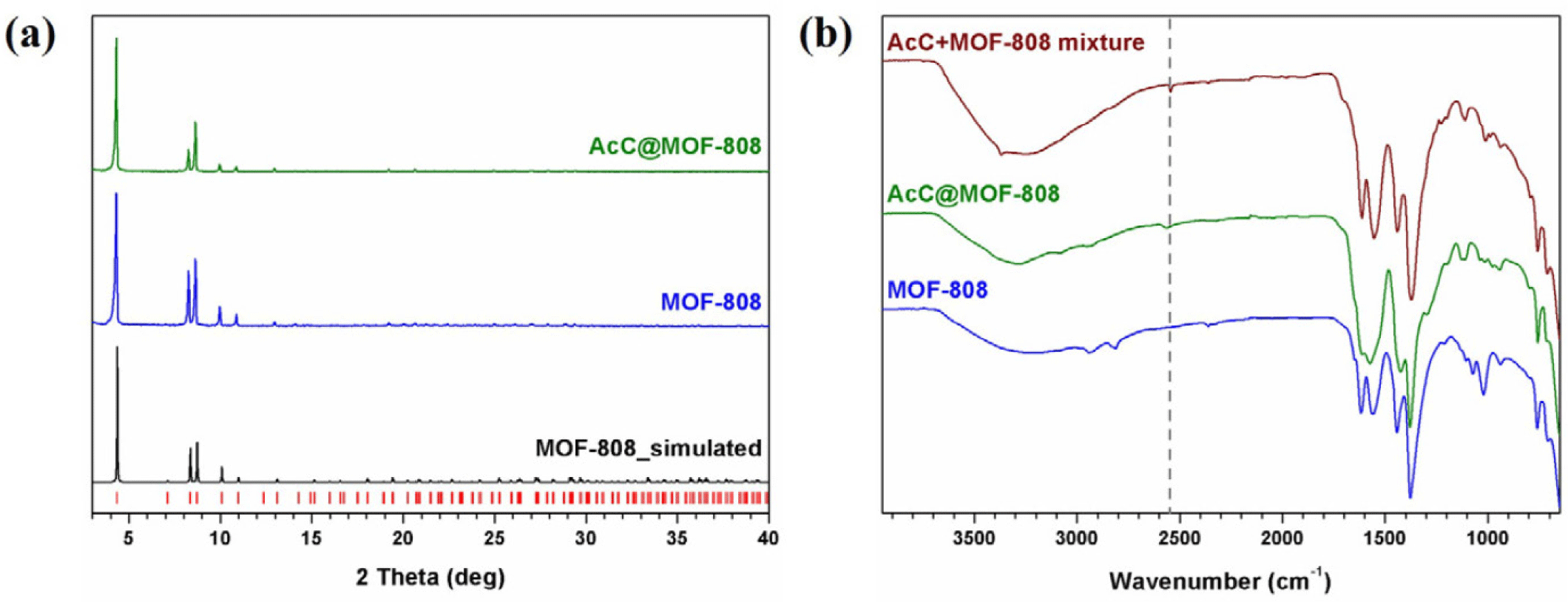
In a 20-mL vial, 100 mg of MOF-808 were dispersed in 5.00 mL of MeOH, followed by the addition of 5.00 mL of 0.300 M AcC in MeOH. After soaking for 12 hours at room temperature, the product was collected by centrifugation and washed three times with fresh MeOH. The washed sample was dried under vacuum at 60°C overnight to remove residual solvent. The crystallinity of AcC@MOF-808 was maintained throughout all the experimental procedures, as confirmed by PXRD (Figs. 2 and 3). The number of AcC integrated within MOF-808 was calculated using 1H NMR spectroscopy of the digested sample (2.00 mg of AcC@MOF-808 dissolved in a mixed solvent of 0.600 mL DMSO-d6 and 50.0 μL of D2SO4) based on the integration of the resonance corresponding to H3BTC and AcC (Supplementary Fig. S1). The morphology and particle size were confirmed by SEM (Supplementary Fig. S2).
To investigate the adsorption behavior of heavy metal ions, Langmuir isotherm model was employed (Eq. 1) [36,37]. In a 20-mL vial, 10.0 mL of Pb(NO3)2 or Cd(NO3)2 aqueous solutions at varying initial concentrations (10, 20, 50, 100, 250, 500, 1,000, and 2,000 ppm) were added to 10.0 mg of AcC@MOF-808. After stirring for six hours at room temperature, the mixtures were vacuum filtered, and the filtrates were collected to determine the remaining concentrations of Pb2+ (or Cd2+) using ICP-OES.
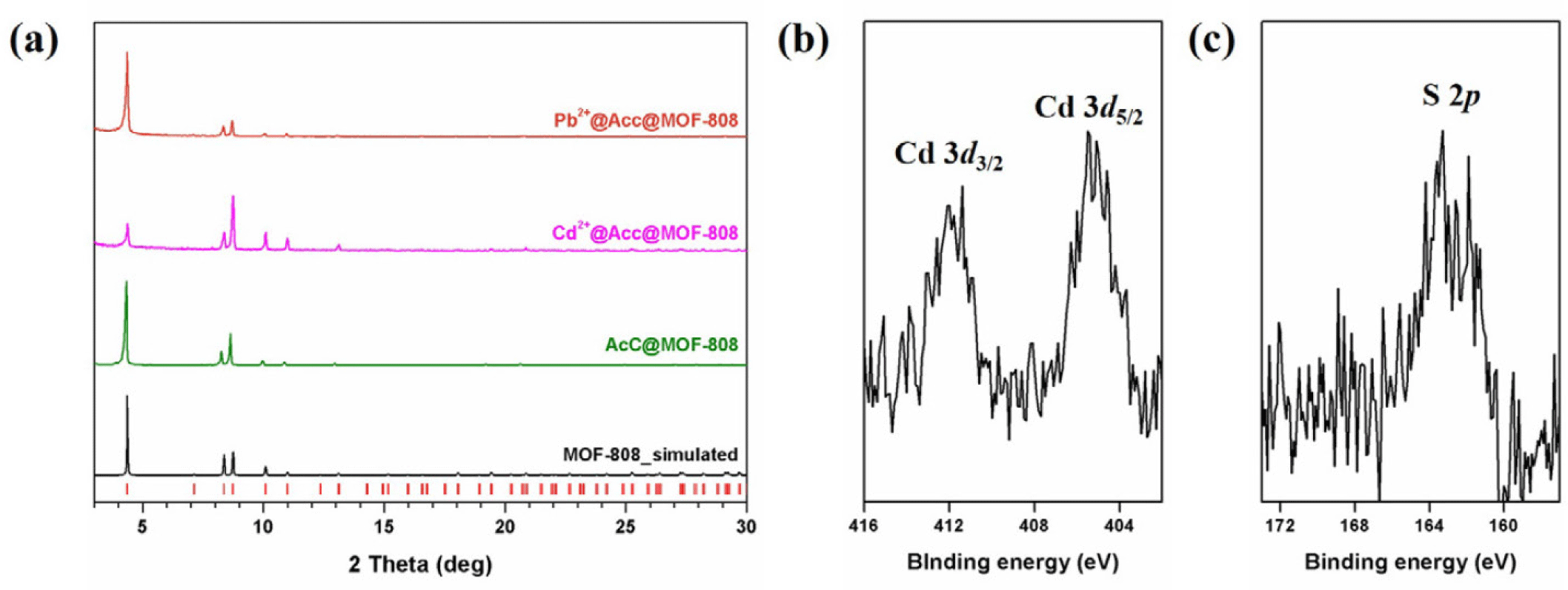
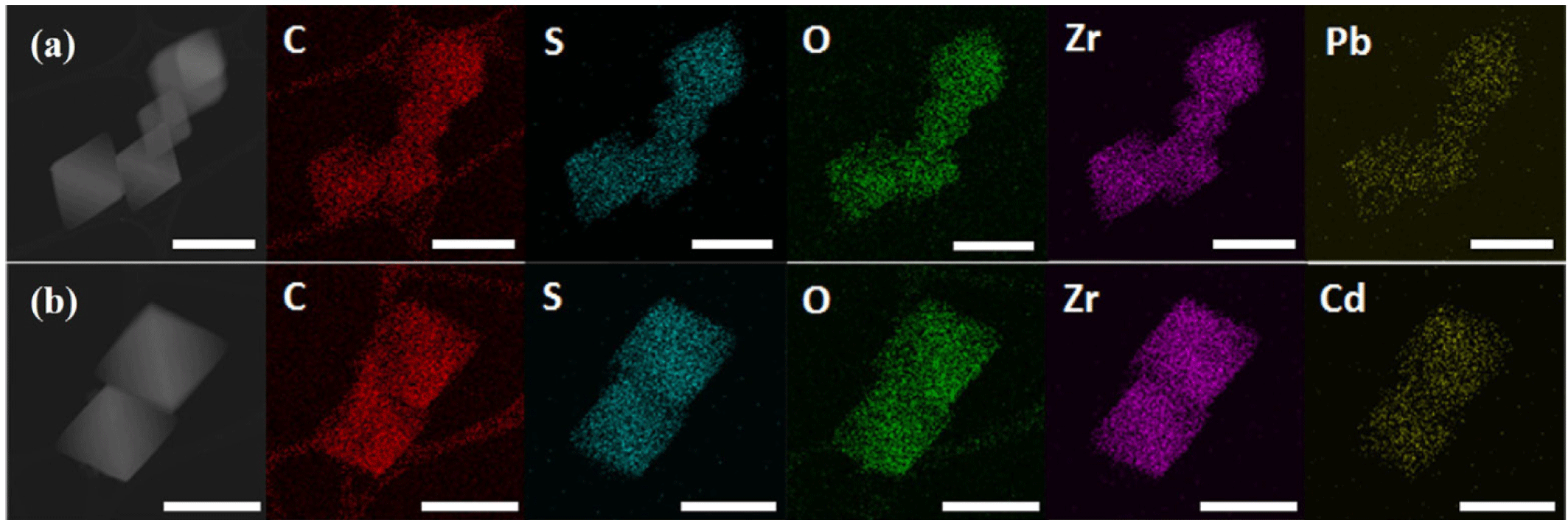
To confirm that heavy metals were adsorbed onto AcC@MOF-808, the sample obtained from the 2,000 ppm adsorption experimental was analyzed. The crystallinity of AcC@MOF-808 was maintained after both Pb2+ and Cd2+ adsorption (Fig. 3). The successful coordination of heavy metals within the framework was further supported by XPS and TEM coupled with energy-dispersive X-ray spectroscopy (TEM-EDS) (Figs. 3 and 4)
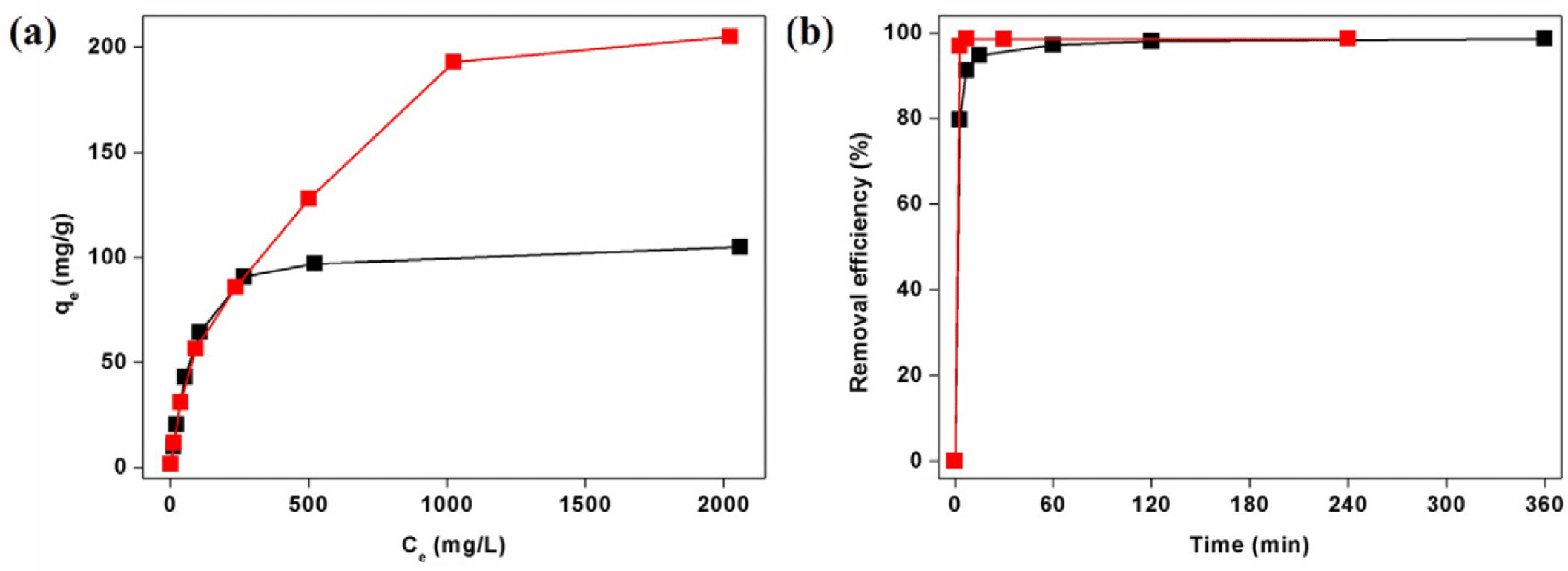
The adsorption capacities and removal efficiencies are presented in Fig. 5a, while the Langmuir model fitting used to determine the maximum adsorption capacities is shown in Supplementary Fig. S4. The experimental data fit the Langmuir model well, with correlation coefficients (R2) of 0.999 for both Pb2+ and Cd2+, allowing reliable estimation of the maximum adsorption capacities (qmax).
The linearized form of the Langmuir isotherm equation is given as:
where Ce (mg L−1) and qe (mg g−1) represent the equilibrium concentration and adsorption amount of M2+ (M=Pb and Cd), respectively; qmax (mg g−1) is the maximum adsorption capacity, and KL (L mg−1) is the Langmuir constant.
To analyze the adsorption kinetics, the pseudo-second-order kinetic model was applied (Eq. 2) [36,37]. In a 20-mL vial, 10.0 mL of Pb(NO3)2 or Cd(NO3)2 aqueous solutions (10 ppm) were added to 10.0 mg of AcC@MOF-808. The mixtures were stirred for different time intervals ranging from three to 360 minutes at room temperature, then vacuum filtered, and the filtrates were collected to determine the remaining concentrations of Pb2+ and Cd2+ using ICP-MS.
The experimental kinetic data are presented in Fig. 5b, and the corresponding pseudo-second-order model fittings are shown in Supplementary Fig. S5 (Eq. 2). The data fitted the pseudo-second-order model well, with R2=0.999 for both Pb2+ and Cd2+, allowing reliable estimation of rate constant (k2).
The linearized form of the pseudo-second-order equation is given as:
where qt (mg g−1) and qe (mg g−1) represent the adsorption amounts of M2+ (M=Pb and Cd) at any time t and at equilibrium, respectively; k2 (g mg−1 min−1) is the rate constant.
Results
To graft thiol groups onto a heterogeneous solid-state adsorbent for heavy metal removal, we employed MOFs as scaffolds [14–16]. Among them, Zr-based MOFs were selected because of their exceptional stability, which arises from the strong coordination between Zr4+ (a hard acid) and carboxylate ligands (hard bases) [31,32]. In particular, MOF-808 was chosen for this study because its Zr6 nodes contain up to six coordination sites occupied by formate ligands that can be exchanged with hydroxyl groups, allowing further functionalization with organic linkers such as amino acids or sulfamic acid [35]. In addition, MOF-808 possesses large pore sizes (11.8 Å and 18.4 Å), providing sufficient space for grafting functional molecules and accommodating heavy metal ions (Fig. 1) [35].
MOF-808 was first synthesized following a modified literature procedure using ZrOCl2∙8H2O, 1,3,5-bezenetricarboxylic acid (H3BTC), and formic acid in DMF at 100°C for 18 hours (see Methods for details) [35]. This resulting product exhibited 400–600 nm octahedral particles without impurities, as confirmed by PXRD and SEM analyses (Figs. 2 and Supplementary Table S1).
Next, to introduce soft-base functional groups, AcC was selected because it contains both carboxylic acid groups (capable of coordinating to the Zr6 nodes) and thiol groups (for binding soft-acid metal ions such as Pb2+ and Cd2+) (Fig. 1). The aliphatic moiety of AcC also enhances its solubility in methanol, facilitating the grafting process. Prior to functionalization, formate ligands on MOF-808 were exchanged with hydroxyl groups through methanol treatment to create accessible coordination sites [35]. Subsequently, MOF-808 was immersed in a methanolic solution of AcC and stirred for 12 hours, yielding AcC@MOF-808 after washing to remove unreacted AcC (see Methods for details).
PXRD and SEM analyses confirmed that AcC@MOF-808 retained the crystallinity and morphology of the parent MOF, with no observable impurities (Figs. 2 and Supplementary Table S1) To determine the number of AcC molecules coordinated to each Zr6 node, digestion NMR spectroscopy was performed, revealing that five AcC molecules were grafted per Zr6 node, corresponding to 83% of the available coordination sites (Supplementary Fig. S2). The N2 adsorption isotherms (77 K) showed a noticeable decrease in adsorption capacity for AcC@MOF-808 relative to pristine MOF-808, consistent with the successful incorporation of AcC within the framework (Supplementary Fig. S3). In addition, the Brunauer-Emmett-Teller (BET) surface areas and pore volumes decreased after AcC grafting, consistent with the N2 adsorption isotherm results, indicating that AcC occupies the internal pore volume of MOF-808 (Table S1).
The presence of free thiol (–SH) groups after functionalization of MOF-808 to AcC@MOF-808 was confirmed by FTIR spectroscopy, which showed a distinct –SH stretching band from AcC at 2,563 cm−1, also observed in the physical mixture of MOF-808 and AcC for comparison (Fig. 2).
Having established the successful incorporation of AcC and the presence of accessible thiol sites, we next evaluated the heavy metal binding performance of AcC@MOF-808 toward Pb2+ and Cd2+ ions as a model system for wastewater purification. As a point of comparison, it has been reported that pristine MOF-808, which lacks thiol groups, does not adsorb Pb2+ or Cd2+ [38]. To determine the maximum adsorption capacity for heavy metal ions, 1,000 ppm aqueous solutions of Pb(NO3)2 and Cd(NO3)2 were prepared. Then, 10 mg of AcC@MOF-808 was added to each metal solution, corresponding to 100 equivalents of metal ions per MOF. The mixtures were stirred for six hours to ensure saturation of metal uptakes. After soaking, we measured ICP-OES after filtration to check Pb and Cd amounts for MOF precipitants (Supplementary Fig. S4). To calculate the potential maximum uptake of AcC@MOF-808, Langmuir isotherm model was performed, and we found that AcC@MOF-808 were able to uptake maximum 231 mg g1 for Pb2+ and 109 mg g−1 for Cd2+(Supplementary Fig. S4) [36,37]. After soaking in 1,000 ppm heavy metal solutions, AcC@MOF-808 were both maintain their crystallinity in Pb2+ and Cd2+ solutions, which exhibited the potential for sustainable solid-state adsorbents (Fig. 3). In addition, after exposure to Cd2+ solution, AcC@MOF-808 maintained their thiol group 163 eV and contained Cd2+ at 405 eV and 411 eV by confirming XPS results (Fig. 3).
To confirm the metal ion adsorption occurred not only on the surface but also within the internal pores of AcC@MOF-808, TEM coupled with energy-dispersive X-ray spectroscopy (EDS) was performed on AcC@MOF-808 samples after Pb2+ and Cd2+ adsorption (Fig. 4). The TEM-EDS elemental mapping of both Pb- and Cd-loaded AcC@MOF-808 revealed homogeneous distributions of Pb and Cd throughout the particles. The uniform overlap of Pb/Cd with Zr and S signals indicates that Pb2+ and Cd2+ ions were bound to the thiol groups uniformly across the entire framework, demonstrating efficient diffusion and adsorption throughout the MOF structure (Fig. 4).
To evaluate the removal efficiency of AcC@MOF-808 under realistic conditions, we tested its performance for the adsorption of trace concentrations of heavy metal ions, as typically found in contaminated drinking water systems [1–3]. For this purpose, 10 ppm aqueous solutions of Pb2+ and Cd2+ were prepared and treated with AcC@MOF-808 as a solid-state adsorbent (Fig. 5). AcC@MOF-808 rapidly removed Pb2+, achieving 97% removal within three minutes and 99% within seven minutes. Cd2+ adsorption was slightly slower, with 95% removal at 15 minutes and 99% after three hours, demonstrating the high adsorption efficiency of AcC@MOF-808 even at low metal concentrations.
To further investigate the adsorption mechanism, kinetic studies were carried out using the pseudo-second order model and the experimental data fitted this model well, with correlation coefficients (R2) of 0.999 for both Pb2+ and Cd2+(Figs. 5b and Supplementary Fig S5) [36,37]. Interestingly, we found that the rate constants (k) for both metal ions exhibited relatively fast adsorption kinetics compared to other MOF-based adsorbents [16]. This enhanced rate is attributed to the flexible aliphatic fragment of AcC, which allows greater molecular mobility and increased the probability of thiol–metal binding. The rate constant for Pb2+ adsorption was calculated as 6.6×103g mg−1 min−1, comparable to previously reported thiol-functionalized adsorbents with aliphatic moieties, such as SiO2–Al2O3–Silane-SH (k= 3.7×103g mg−1 min−1) [39]. Similarly, the k value for Cd2+ adsorption was 1.1×103g mg−1 min−1, in line with reported values for other thiol-based Cd2+ adsorbents (e.g., SiO2–Al2O3–Silane-SH, k = 4.7×103g mg−1 min−1) [39].
In addition, these adsorption rates are significantly faster than those reported for thiol-based amino acid-grafted MOF-808 materials (MOF-808@Msc and MOF-808@Cys), which require more than 200 minutes for heavy metal uptake [38]. Notably, AcC@MOF-808 achieves Pb2+ adsorption approximately 20 times faster while also exhibiting higher capacities than these materials (MOF-808@Cys: 175 mg g−1 for Pb2+ and 40 mg g−1 for Cd2+; MOF-808@Msc is unstable under acidic conditions), owing to the presence of the aliphatic fragment and acetyl groups in AcC [36]. These results confirm that AcC@MOF-808 is a highly effective adsorbent for heavy metal ions and has strong potential for practical water purification applications.
AcC@MOF-808 exhibited highly efficient adsorption of Pb2+ and Cd2+ ions. To further evaluate its selectivity toward these soft-acid heavy metal ions, competitive adsorption experiments were performed using other divalent metal ions commonly found in real water systems, including Zn2+, Ca2+, and Mg2+ [14–16]. Under the same conditions used for Pb2+ and Cd2+ adsorption, 10 ppm aqueous solutions of Zn(NO3)2, Ca(NO3)2, and Mg(NO3)2 were prepared and tested with AcC@MOF-808 as the adsorbent. The removal efficiencies were 19% for Zn2+, 6% for Ca2+, and 2% for Mg2+ (Fig. 6).
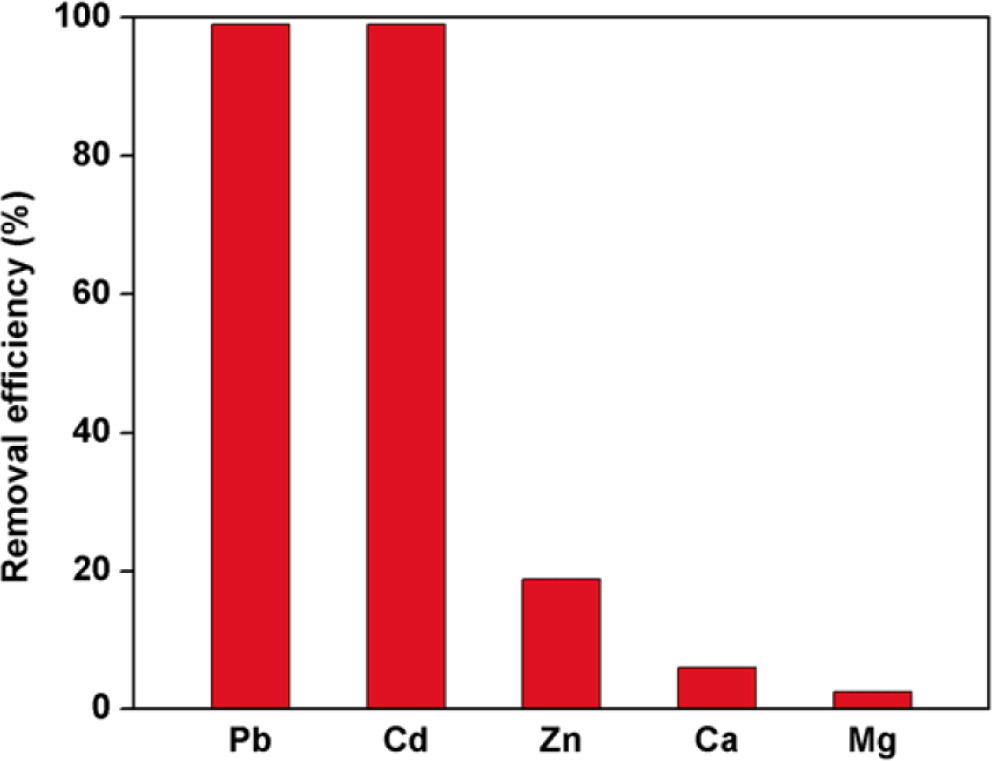
These results indicate that AcC@MOF-808 shows strong selectivity for soft-acid metal ions (Pb2+ and Cd2+) over hard-acid ions (Ca2+ and Mg2+) (Fig. 6). The moderate adsorption of Zn2+ can be attributed to its intermediate acid-base character, which allows partial interaction with the thiol groups of AcC, but still much weaker than that observed for Pb2+ and Cd2+ (Fig. 6). Overall, the strong preference for Pb2+ and Cd2+ confirms that the thiol-functionalized MOF framework acts as an effective and selective solid-state adsorbent for soft-acid heavy metal ions.
In conclusion, we successfully demonstrated the grafting of AcC, which contains thiol functional groups with aliphatic fragments and acetyl groups, onto MOF-808 to achieve selective and rapid adsorption of heavy metal ions (Pb2+ and Cd2+) over other divalent ions (Zn2+, Ca2+, and Mg2+) in aqueous systems. AcC was coordinated to 83% of the available sites of Zr6 clusters through carboxylate linkages, while the resulting AcC@MOF-808 retained its crystallinity, morphology, and porosity, enabling a high density of accessible thiol sites within the framework. Owing to the high grafting efficiency, the maximum adsorption capacities for Pb2+ and Cd2+ reached 231 mg g−1 and 109 mg g−1, respectively. Spectroscopic and microscopic analyses confirmed that heavy metal ions were uniformly adsorbed throughout the framework without structural degradation. At trace concentrations (10 ppm), AcC@MOF-808 achieved remarkable removal efficiencies of 99% for Pb2+, including rapid uptake within the first seven minutes, and Cd2+, while showing minimal uptake for competing ions (Zn2+, 19%; Ca2+, 6%; Mg2+, 2%), consistent with the HSAB principle. The adsorption kinetics followed a pseudo-second order model, indicative of a chemisorption mechanism, with rate constants (k) of 6.6×103 g mg−1 min−1 for Pb2+ and 1.1×103 g mg−1 min−1 for Cd2+. These results demonstrate that AcC-functionalized MOFs serve as robust, selective, and eco-friendly solid-state adsorbents for efficient heavy metal removal from aqueous environments.
Looking forward, this study can be extended by incorporating other amino acid-derived ligands with diverse functional groups to fine-tune the pore environment and metal ion selectivity of MOFs. Such eco-friendly frameworks could also serve as promising candidates for catalysis or adsorption of larger hazardous organic molecules. Further optimization toward enhanced reusability, stability, and scalable synthesis would accelerate the practical application of amino acid-functionalized MOFs in real wastewater purification and environmental remediation systems.

